By Dr. Karishma S. Kaushik, MBBS, MD, PhD
Savitribai Phule Pune University, Pune, India

The beauty of biofilms
Do you recall studying microbes in school and university textbooks and laboratories? You probably remember them as illustrations of single-celled organisms or as free-floating forms in nutrient-rich broths. Turns out that, in most real-world conditions, microbes adopt a far more complex lifestyle. In the human body, microbes such as bacteria and fungi, exist as complex, multicellular communities known as biofilms. Biofilms are three-dimensional structures, often seen as aggregates of microbes in self-produced polymeric matrix, attached to each other or to a surface. In biofilms, microbes actively communicate with each other via chemical signals that increase with cell density. This cell-to-cell signaling or quorum sensing enables communication between diverse microbial species, mediating a range of processes and behaviors. This is important given that biofilms are nearly always found as mixed-species communities, consisting of diverse bacterial and fungal species existing in close proximity with each other. Further, biofilms possess distinct nutrient acquisition and protection mechanisms, contributing to their extended survival and persistence.
When the beauty turns into a beast
The clinical relevance of multi-species biofilms is apparent, ranging from the relatively innocuous dental plaque, to limb-threatening wound infections, and life-threatening lung and medical device infections. Notably, clinical biofilms are notoriously tolerant to antimicrobial treatments, often prompting long-term antibiotic usage with uncertain reasons for failure. Recognizing this, there has been an increased interest in understanding biofilms, particularly their formation and persistence under human conditions [1]. This could lead to developing effective therapeutic and preventative approaches, including non-conventional and combinatorial treatments. So far, a large component of biofilm research has used laboratory systems, such as polystyrene surfaces and protein broths, in an attempt to study biofilms and investigate treatment effects. While this has moved the field forward, a major concern is that these conditions poorly replicate the human host environment, and therefore may not faithfully mimic biofilms in infection states. In clinical conditions, biofilms exist in close proximity with a range of host components, such as cells, tissues, and body fluids. Together, these microbial and host factors constitute the infection microenvironment, with specific biochemical and biophysical signatures. The presence of these varied factors is likely to affect biofilm features such as formation and composition, and influence response to treatments. Given this, there is an increased push to incorporate host relevant components in biofilm studies, that can closely mimic clinical conditions and provide hitherto unexplored insights into biofilm infections [2].
How can we better understand the beast?
My research group aims to understand biofilm infection microenvironments, with a focus on studying wound biofilms under host-relevant conditions. Chronic skin wounds are a major healthcare burden, often afflicting individuals with long-standing diabetes mellitus, and can result in hospitalization, amputation and even death. Bacterial biofilms are the single-most important cause of non-healing wounds. In wounds, biofilms are most often mixed-species infections, and Staphylococcus aureus and Pseudomonas aeruginosa are the most common co-pathogens. These mixed-species biofilms interact with, and are influenced by, various host components such as the wound bed (consisting of skin cells fibroblasts and keratinocytes), presence of wound fluid (a protein-rich drainage), chemical factors (lactic acid, glucose, enzymes) and immune cells (neutrophils, macrophages). Wound biofilms are recalcitrant to conventional antibiotics and immune clearance, which further fuels the chronic infection state. We study wound biofilms under host-relevant conditions, by developing biomimetic platforms that recapitulate the infection microenvironment. These laboratory systems incorporate select host components, and thereby enable human-relevant insights into the formation and features of wound biofilms. In contrast to animal models of biofilm infections, these platforms offer the advantages of selective and precise control, in an ethical and cost-effective manner. In doing so, they hold potential to serve as preclinical drug discovery platforms that lend well for accelerated evaluation of wound biofilm diagnostics and novel anti-biofilm therapeutics.
Based on clinical wound fluid composition, we have developed a laboratory formulation of a wound milieu (in vitro wound milieu, IVWM) that includes, in addition to serum (to recapitulate wound fluid), wound matrix proteins (collagen, fibrinogen, fibronectin) and biochemical factors (lactic acid, lactoferrin). Mixed-species biofilms of S. aureus and P. aeruginosa formed in this milieu recapitulate widely reported clinical biofilm features such as 3D structure, interspecies interactions and increased antibiotic tolerance, 3D structure, and interspecies interactions [3]. Further, the wound milieu is simple to formulate, uses laboratory-grade components, and is compatible with standard biofilm assays. In partnership with industry, we are currently leveraging this milieu to validate wound biofilm diagnostics. In doing so, this human-relevant wound milieu holds potential to fast-track therapeutic and diagnostic interventions, which is a significant step forwards from minimalistic laboratory models and animal studies. In addition to being bathed in wound fluid, wound biofilms exist in proximate association with the wound bed, comprised of host cells such as epidermal keratinocytes and dermal fibroblasts. To better mimic host-biofilm interactions, we are building wound biofilm platforms with cellular elements, that will serve as preclinical testing systems for anti-biofilm therapeutics.
Images and descriptions
Image 1: Microscopic visualization of mixed-species biofilms of S. aureus (green) and P. aeruginosa (red) grown in laboratory broth (LB), serum (Fetal Bovine Serum, FBS) and in vitro wound milieu (IVWM). Notably, the structure and composition of the mixed-species biofilms in the IVWM is distinct from that in serum alone, underscoring the importance of host elements such as proteins and biochemical factors [3]. Photo credit: Snehal Kadam
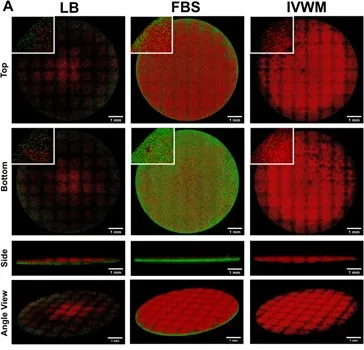
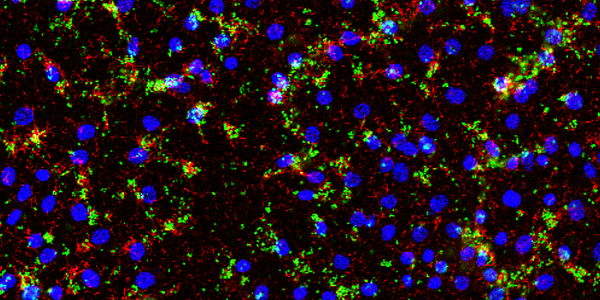
Image 2: Microscopic visualization of biofilms of S. aureus (green) and P. aeruginosa (red) grown on epidermal keratinocytes showing mixed-species biofilm aggregates growing in association with the host cells (seen as blue nuclei), that resembles biofilms seen in clinical wounds (unpublished, Kaushik KS). Photo credit: Radhika Dhekane

Select references
[1] Costerton JW, Lewandowski Z, Caldwell DE, Korber DR, Lappin-Scott HM. Microbial biofilms. Annu Rev Microbiol (1995).
[2] Roberts AEL, Kragh KN, Bjarnsholt T, Diggle SP. The limitations of in vitro experimentation in understanding biofilms and chronic infections. J Molecular Biology (2015).
[3] Kadam S, Madhusoodhanan V, Dhekane R, Bhide D, Ugale R, Tikhole U, Kaushik KS. Milieu matters: An in vitro wound milieu to recapitulate key features of, and probe new insights into, mixed-species bacterial biofilms. Biofilm (2021).