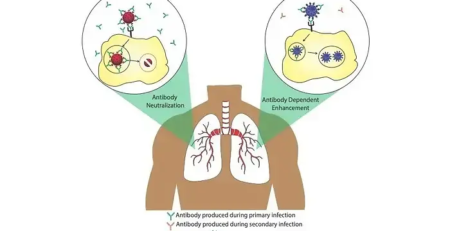
Vaccines work by stimulating the immune system using an attenuated/inactivated virus or subunit molecules, thereby training the immune system to recognize and act against similar molecules in the future. But the immune response to a vaccine should not result in manifestations of symptoms of the disease. Antiviral vaccines activate both arms of the adaptive immune response (immunity acquired as a result of exposure to an antigen), and innate immune response (first line of defence mediated by physical, chemical and cellular defences) in an individual. Vaccines can trigger the production of antibodies, opsonization & phagocytosis (a process by which foreign pathogens are tagged for elimination by phagocytes), and antibody dependent cell mediated cell cytotoxicity (mechanism through which immune cells recognise and kill cells coated with antibodies).

Vaccines are developed in two stages, a pre-clinical stage that involves in vitro testing and animal testing, and a clinical development stage that involves human trials. The clinical development stage can be spilt into four phases that end with the introduction of the vaccine in the market, making it accessible to the public. The duration of the whole process of vaccine development can range anywhere between a year or two to a decade or more. Although it is possible to create a vaccine in less than a year, it cannot be deployed until it has undergone extensive human clinical trials (development), the duration of which can vary depending on success observed in each clinical phase. This is very challenging during a pandemic. 67 vaccines are in currently in pre-clinical development for COVID-19. But vaccines that can be developed relatively fast are DNA and RNA vaccines. Two of the three vaccines currently in clinical development are of this kind. (Source: WHO). The National Institutes of Health (NIH)/Moderna’s mRNA 1273 and CanSino Biological Inc./Beijing Institute of Biotechnology’s adenovirus Type 5 vector (Ad5-nCoV) vaccines are in Phase I clinical trials. The success of a vaccine depends on its ability to induce an immune response in the human body and the mutagenic capacity of the virus it targets. Some vaccines can have local, systemic, and/or allergic adverse reactions.

Before Edward Jenner popularised the term ‘vaccination’ and devised a method to gain immunity against smallpox, the disease had killed countless people with a mortality rate of 20-30%. Subsequently, smallpox was completely eradicated in December 1979, owing to a global vaccination outreach program by the WHO. Likewise, vaccines have been successful against other viral infections as well, to name a few – Human Papilloma, measles and varicella. From the existing data, the mortality rate (number of deaths divided by the reported cases) for SARS-CoV-2 is 3-4% (Source: WHO). Will we be able to overcome this pandemic? We hope that the heightened worldwide research initiatives for COVID-19 will lead to the successful identification of a vaccine candidate before the end of 2020.
References
- DRAFT landscape of COVID-19 candidate vaccines –WHO report dated 20th March 2020.
- Nicole Lurie, M.D., M.S.P.H., Melanie Saville, M.D., Richard Hatchett, M.D., and Jane Halton, A.O., P.S.M. Developing Covid-19 Vaccines at Pandemic Speed. NEJM. March 30, 2020. DOI: 10.1056/NEJMp2005630.