The vast majority of individuals affected by rare diseases in India continue to live without access to effective therapies, as curative or disease-modifying treatments remain largely unavailable. Even when such therapies exist, the high-cost places them out of reach for most patients. Developing effective treatments for rare diseases requires a comprehensive and coordinated approach—one that includes the creation of patient registries, development of natural history models tailored to the Indian population, advancement of enabling technology platforms, and the design of novel, intellectually protection free drug molecules.
Strengthening indigenous manufacturing capabilities for rare disease therapeutics is critical to ensuring affordability and accessibility. There is a need for a conducive regulatory environment that facilitates the efficient conduct of clinical studies needed for drug approvals. However, integrating the multiple facets of rare disease management remains a significant challenge, as these conditions often present unique complexities not typically encountered in more common diseases.
A major barrier to the development of advanced therapies—particularly for rare genetic disorders—is the absence of clear regulatory pathways and guidance for drug development and approval process in India. The small patient populations, coupled with limited natural history data and a lack of insight into disease-specific needs, make it especially difficult to translate laboratory research into viable clinical interventions.
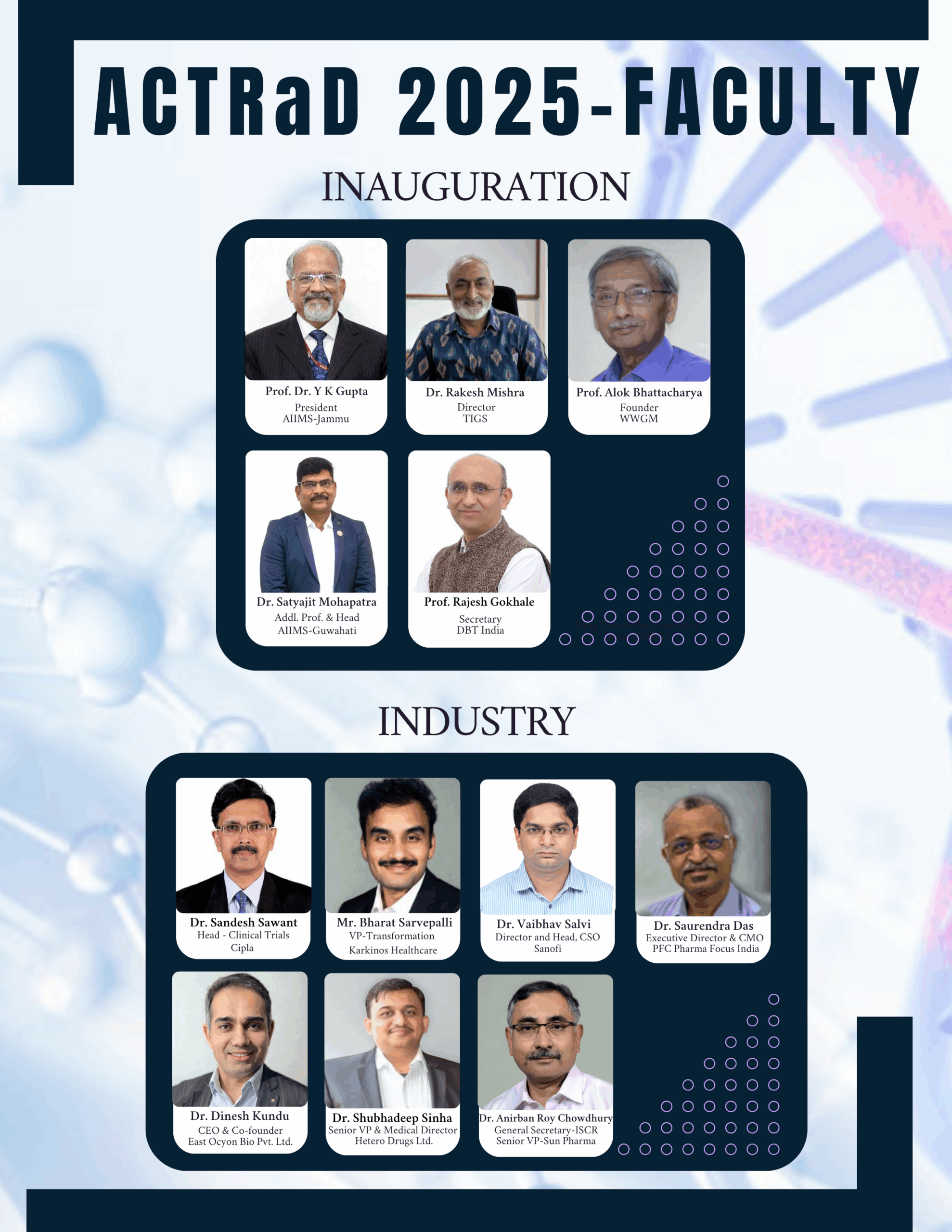
To overcome these challenges, there is a pressing need for greater sensitization and collaboration among all stakeholders, including researchers, clinicians, regulators, industry, and patient advocacy groups. Such coordinated efforts will be pivotal in enabling clinical trials for rare diseases and accelerating the approval and availability of life-saving therapies.
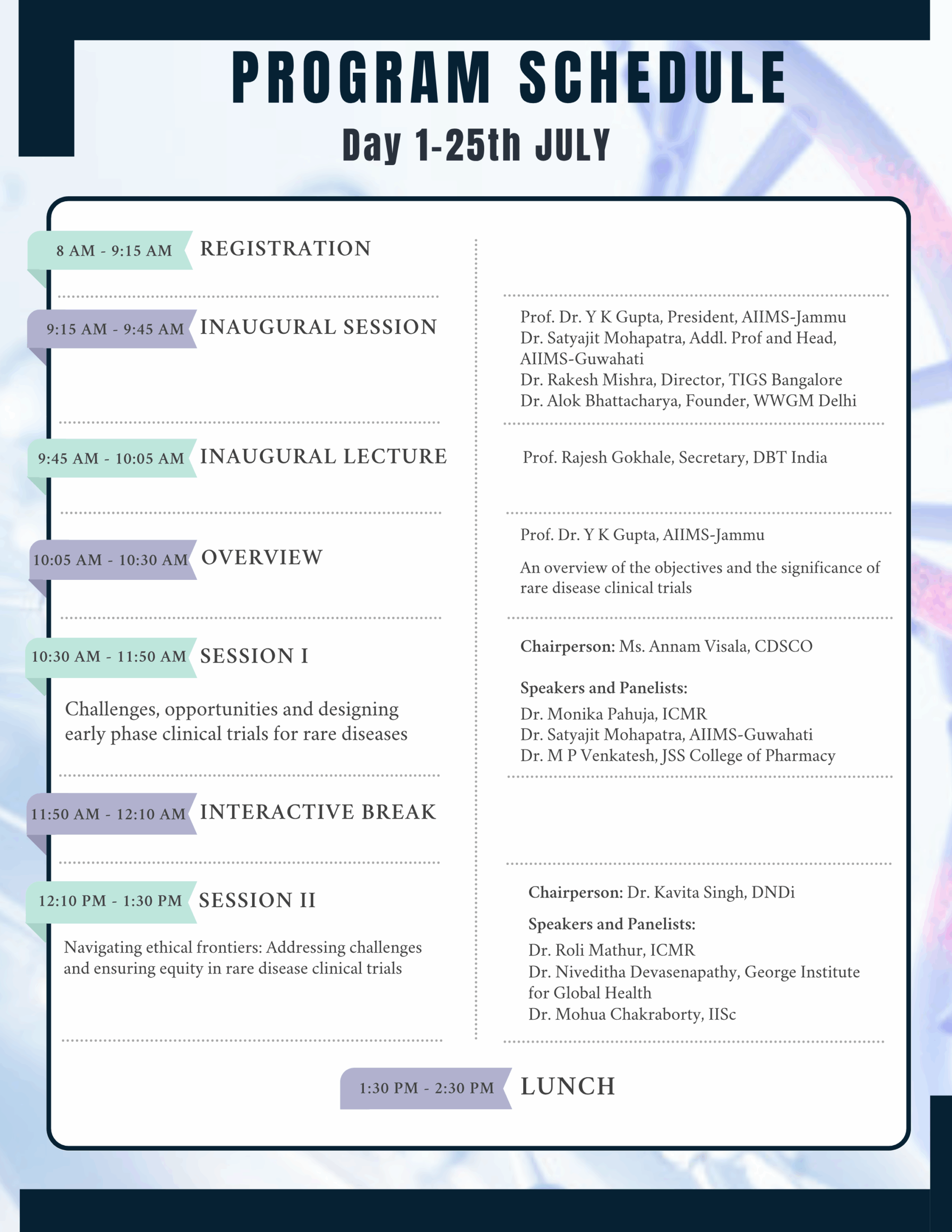
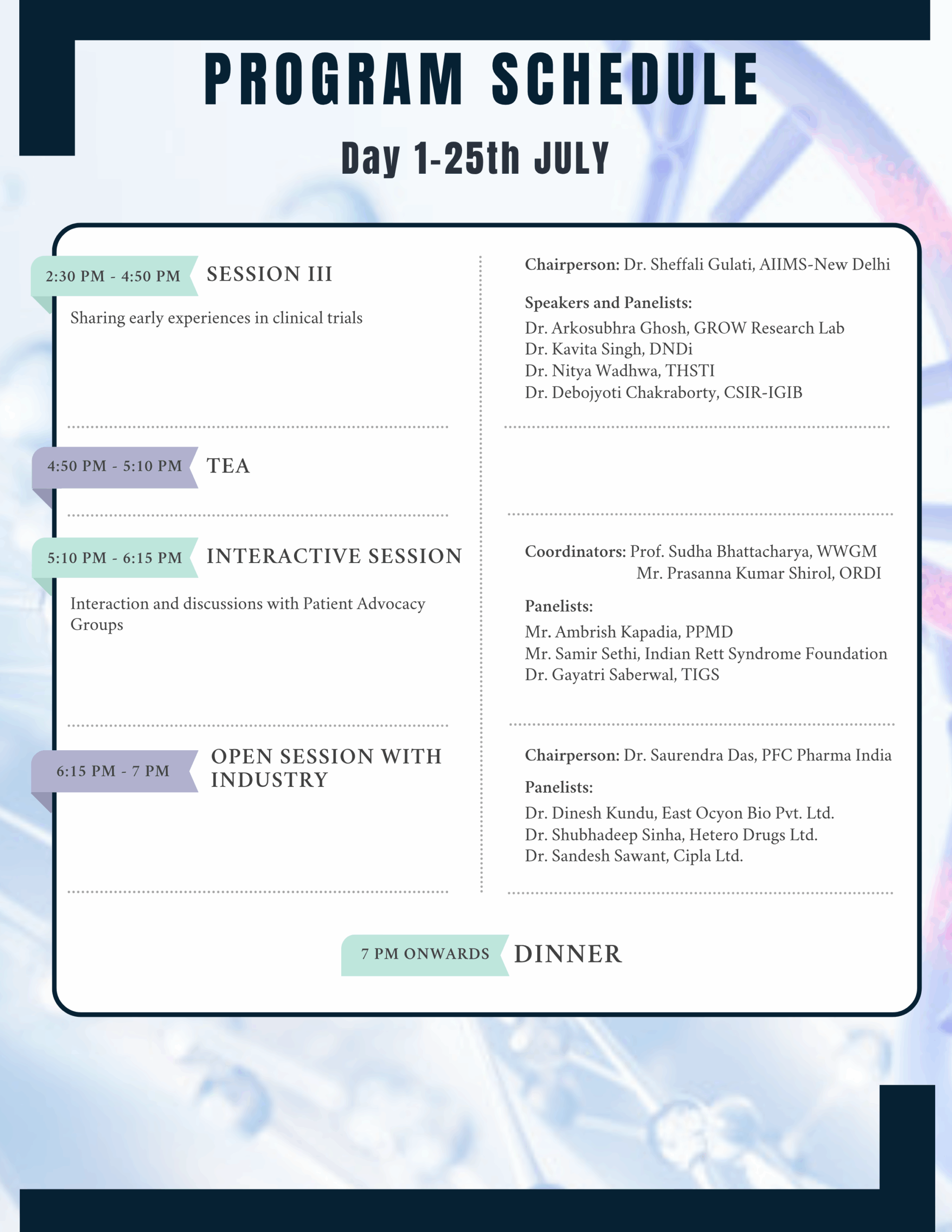
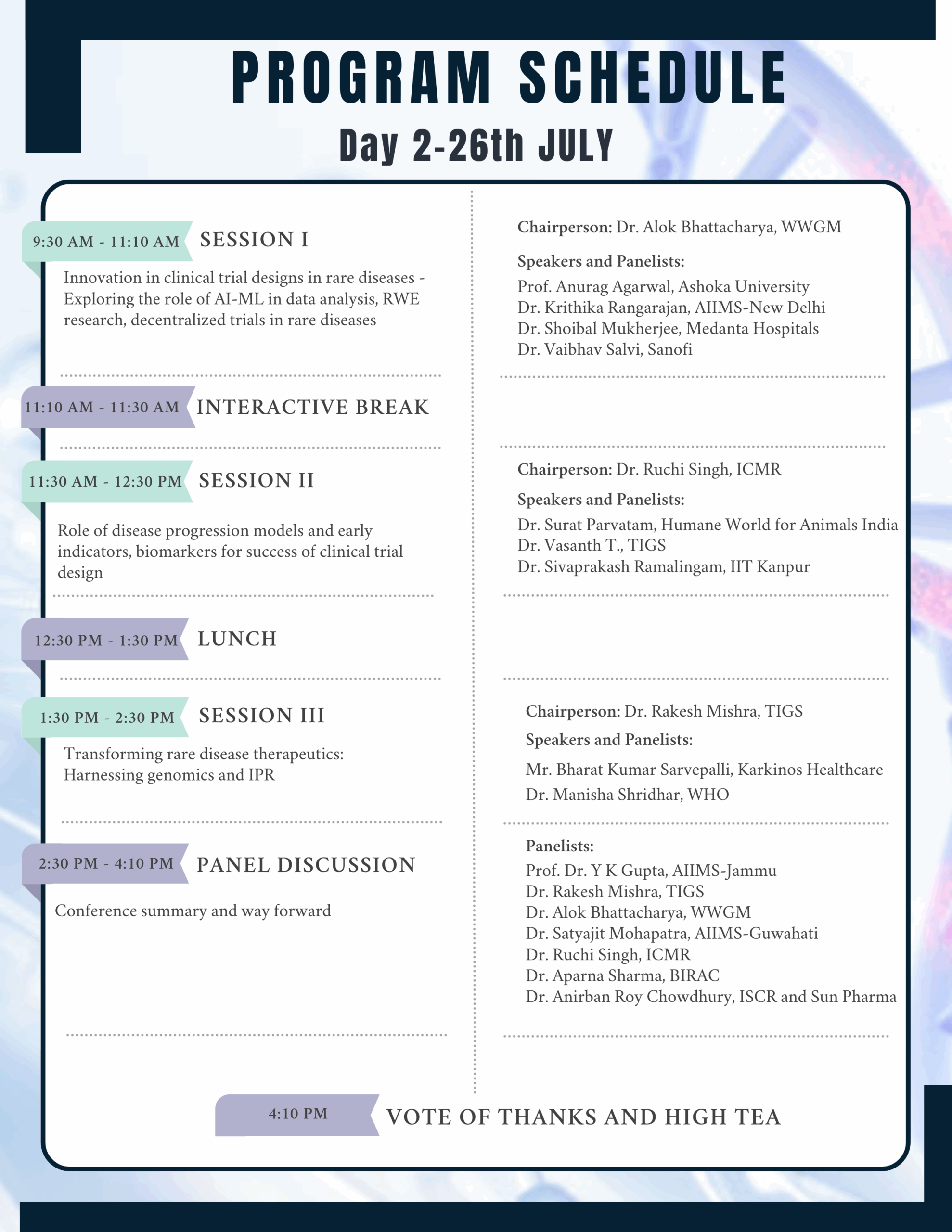
In this regard a National Conference on ‘Advancing Clinical Trials in Rare Diseases (ACTRaD)’ is being organised on July 25-26, 2025, at the Indian National Science Academy, New Delhi. We aim is to produce a short guidance document that can help all stakeholders engaged in the area of clinical trails and rare diseases.
Organizers:
Tata institute for Genetics and Society, Bengaluru
World Without GNE Myopathy (WWGM), New Delhi
AIIMS Guwahati
YKG Academy, New Delhi
Organization for Rare Diseases India, Bengaluru
For complete program schedule – Click here