By Shweta Yadav
Vertebrates, including humans, have a complex immune system, which constantly functions to protect them from harmful foreign agents including bacteria and viruses. One of the major defence arms (humoral immunity) of the body comprises of special proteins called antibodies or immunoglobulins. These are produced in response to bacterial or viral infections and are crucial for limiting or even resolving infections at times. They are ‘Y’ shaped proteins synthesized by specialized cells (called plasma cells) of the immune system and are capable of binding to the surface regions (epitopes) of invading pathogens. Unlike skin, which is also a primary defence mechanism that can defend us against all kinds of pathogens, antibodies are specifically produced against a given epitope and hence are specific to a particular pathogen, more like a lock and key. Topologically, antibodies have two binding sides; the top portion of the ‘Y’, called Fab (comparable to the part of the key that fits into the lock), is variable in nature and binds to specific epitopes, and the bottom portion, called Fc (more like the part of the key we hold while operating the lock), is constant and binds to receptors present on the surface of immune cells like macrophages and monocytes.
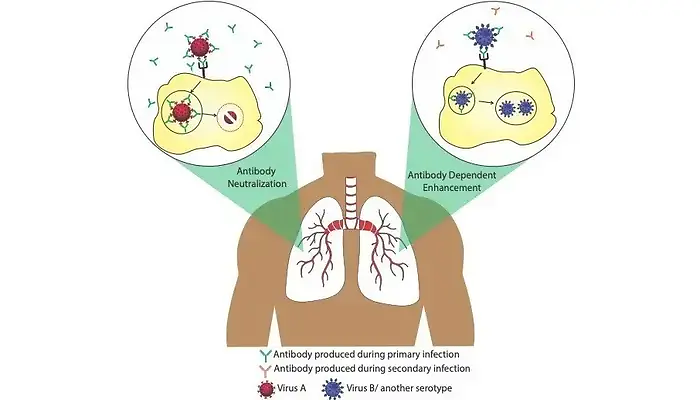
Antibodies are of several kinds (IgG, IgM, IgA, IgE and IgD) depending on when and where they are produced in the body post infection. Most infections create an immunological memory in our body so as to be prepared for subsequent infections from the same or similar pathogens. Antibody memories are stored in special cells called memory B-cells that can last for decades. These memory B-cells are the reason several childhood vaccines protect us for life. Antibody response plays a crucial role during viral infections. Plasma cells produce several antibodies targeted against multiple viral epitopes. Although all of them have Fab capable of binding to various parts of the virion, only a subset of these antibodies are able to block the infection. These are referred to as neutralizing antibodies. Antibodies can neutralize viral infections in a number of ways. For example, they could interfere with virion binding to cell surface receptors, block their uptake into cells, prevent uncoating of their genomes in endosomes, or cause aggregation.
The beneficial roles of antibodies are well known, however, sometimes the invading viruses are able to exploit these antibodies to facilitate their own entry into cells and hence result in enhanced infection. This phenomenon is referred to as “Antibody Dependent Enhancement” (ADE) or simply “Immune Enhancement”, and was proposed early in the 1960s by Dr. Scott Halstead. ADE is induced in the presence of either ‘non-neutralizing’ antibodies, which are able to bind to viral particles but not able to cause their destruction, or ‘sub-neutralizing’ antibodies, which are present in insufficient numbers. Such a situation typically arises when antibodies are produced against a particular virus during a primary infection event followed by a secondary infection later, by similar viruses sharing partially similar epitopes (e.g different serotypes of a given virus) or other viruses having similar epitopes. ADE has been observed in case of a variety of viruses, most notably flaviviruses (e.g., dengue virus), HIV, and Ebola viruses. It was first proved experimentally in in-vitro studies done on West Nile fever virus and was subsequently demonstrated for an ever-growing number of human and animal disease-causing viruses. Genome-wide transcriptomic studies suggest that ADE also suppresses the operation of anti-viral pathways in immune cells, thereby enhancing disease manifestations. Due to these reasons, ADE has been a serious concern in the field of antibody-based drug therapy and vaccine design.
Vaccines are substances that mimic an infection by activating our immune cells and thereby creating a defence memory. Vaccines have been helpful in protecting humans from several infectious diseases (e.g. mumps, measles, chickenpox, influenza, hepatitis A, polio etc.) and eradicating some of these diseases altogether (e.g. smallpox and rinderpest). Though vaccines work well for many viral diseases where the pathogen does not evolve much, it is inefficient or requires seasonal doses for viruses that evolve rapidly (e.g. flu causing viruses). Fast evolution is more common in viruses containing RNA as their genome than those containing DNA. One good example is the influenza virus that causes the common flu. Antibody memory formed during one flu season is ineffective during the next outbreak. These evolving viruses are a major concern during vaccine development. An important example of ADE affecting anti-viral vaccine development is the case of dengue fever. The dengue causing virus is known to have four antigenically distinct forms called serotypes. ADE was reported in case of dengue infections, where a secondary infection by a different serotype often elicited severe disease manifestations. The impact of this phenomenon was observed during the efficacy trials run with the first recombinant dengue vaccine in 2015. The rate of hospitalisation for dengue was higher in vaccine recipients than among controls. The likely explanation proposed was that the vaccine mimicked a primary infection by one particular serotype and a subsequent exposure to a different serotype of the virus resulted in ADE. Thus, it is extremely important to consider the possibility of ADE induction while developing anti-viral vaccines.
The recent Covid-19 pandemic is caused by another RNA virus, a novel strain of coronavirus called SARS-CoV-2. Covid-19 is the third serious coronavirus induced pandemic in last 20 years, following SARS in 2002-2003 and MERS in 2012. The difficulties raised by the pandemic has called for rapid vaccine development, and so it is important to understand why it takes several years to create a good vaccine. One of the challenges with coronaviruses is that they share high similarity, thereby increasing the chances of cross reaction between antibodies produced in the body, resulting in ADE. ADE has already been reported to hinder vaccine development against SARS and is likely to be a concern now for Covid-19. However, with lessons learnt from related coronaviruses and with the aid of technological advancements, researchers are working hard to design a safe and effective vaccine that could specifically neutralize the SARS-CoV-2 virus without cross-reacting with other similar viruses. The development of such vaccines would profoundly help contain the pandemic.